Next page: The problem of aging
Decline of Function Aging causes a widescale disruption to the functioning of the human body. As people get older their organs begin to struggle with the basic processes to keep them alive and well.
Although the general process of aging affects all tissues, this degeneration does not occur at the same rate throughout the population or even throughout the body. For example, whereas the skeletal muscles begin aging in the 40s, the cells in the lining of the gut do not begin to show signs of aging until one’s 60s on average.
Increasing age-related changes within the various tissues of the body increases the likelihood of age-related disease. This, coupled with the increasing elderly demographic, may result in a demand for medical specialists and specialized therapies that far exceeds the supply. The growing population of Americans over 65 may thus be better served by therapeutics that address the root cause of aging. This type of therapy should be positioned such that it can be applied to the organs or tissues that need it most as people get older.
Different parts of the body age at drastically different rates. The hourglass number represents the approximate age at which organ begins to age. While the skin begins to lose youthful qualities as early as 18 years of age on average, the brain does not show signs of aging until one’s 70s.
Organs may age at different rates but the aging process for all of them is controlled by the individual cells they contain. As cellular function underlies organ function, the developmental processes that stabilize the cells into the organs of the body also are key to their eventual decline. It is thus useful to understand changes in cellular function across the developmental time course to understand how to delay or repair age-related decay.
At the heart of our mission lies a bold vision: to transform health and wellness as we know it. With our groundbreaking technology, we’re not just innovating – we’re revolutionizing. By harnessing the extraordinary power of embryonic and placental genes, alongside the body’s natural healing mechanisms, we’re on a mission to breathe new life into aging cells.
In the cell’s nucleus , enzymes unwind cellular DNA to make an RNA copy. The RNA matures and leaves the nucleus, heading for the cell’s protein-making machinery known as a ribosome. The ribosome compiles amino acids from within the cell to form a protein based on the RNA code.

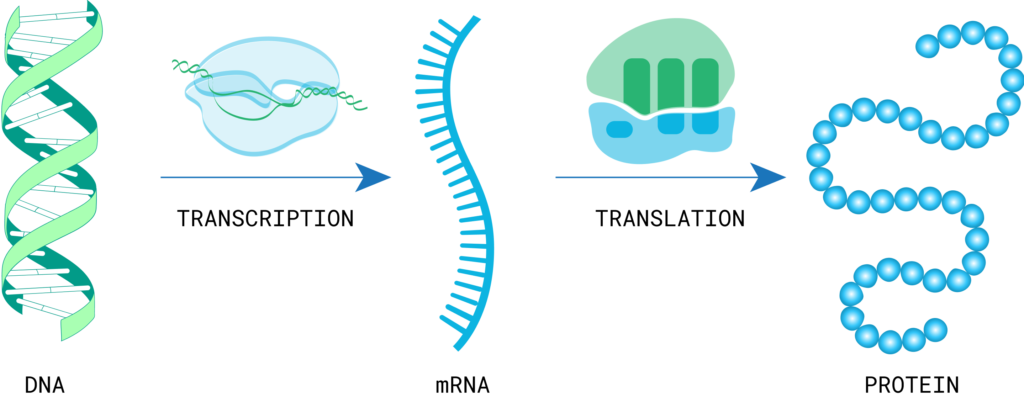
All cells in an individual contain the same sequence of genomic DNA, but it is the specific expression of different pieces of this sequence that determines each cell’s function, type, and location. The process of gene expression is complex, but the most basic explanation is that segments of DNA are read and converted to RNA, which is then read by other machinery in the cell to produce the corresponding proteins.
Expression of different sets of proteins is what enables a single type of embryonic stem cell to differentiate into the wide variety of cell types we see in the body. The differentiation process is tightly regulated in early development such that cells begin to organize from tissue layers into fully integrated organs and the relationships between the cells are generally maintained through adulthood. However, changes in the environment can disrupt these functional relationships within and between cells, leading to disease. Over time, changes in the workings of the cells due to exposure to different medications, foods, sunlight, and other environmental factors begin to build up, disrupting normal cellular and organ function to cause aging.
Understanding how to reverse the epigenetic clock can draw insights from nature’s own mechanisms. During reproduction, the epigenetic slate of an organism is effectively wiped clean through a process known as reprogramming. This process resets the epigenome, allowing offspring to develop from cells that are biologically ageless.
While this total reset is suitable for the formation of a new individual, it is not ideal for anti-aging therapies because it would result in the loss of cellular identity and organizational structure, thereby compromising cellular and organ functions. Instead, a more targeted approach would involve partially reversing the age of cells, all while maintaining their identity
Ananda Labs has pioneered the development of the “Rejuvenation-based Placental Epigenetic Reprogramming” (RPER) technology, utilizing patented placental and embryonic factors. This technology treats cells with a combination of reprogramming factors that shock them out of their aged, dysfunctional states, and promote a more youthful, tightly regulated epigenome. The RPER involves the use of reprogramming factor RNA encapsulated in a fatty membrane casing or clinical grade adeno-associated viruses (AVVs), which can be specifically directed to particular cellular populations. This targeting mechanism ensures that the RNA/AAV is efficiently absorbed and utilized by the body’s cells. Since the RPER is based on RNA/non integrating technology, it does not alter the DNA but instead enhances its expression capability.
Preclinical studies have demonstrated that partial reprogramming can significantly roll back the epigenetic clock in skin and blood vessel cells. It has also been shown to restore muscle tone, enhance regenerative capacity, and reverse biomarkers associated with osteoarthritis following treatment. Current efforts are focused on optimizing the delivery of this therapy to specific cellular populations in vivo. Through these innovations, RPER represents a transformative approach in the field of anti-aging, offering potential widespread benefits for reversing age-related decline at the cellular level.

The epigenome, in the simplest terms, determines how accessible the DNA is to proteins that control gene expression. Looser DNA is more easily accessed by enzymes that convert it into RNA (which is later transformed into protein), whereas more condensed DNA blocks these enzymes, preventing them from generating RNA from certain DNA segments.In normal conditions, the epigenome is able to respond to environmental cues to allow for changes in gene expression that maintain normal cellular function. As the cell ages, the epigenome loses this flexibility, increasing the likelihood of inappropriate gene expression. We can measure this rigidity to get an approximation of cell age using an epigenetic clock.
A person’s epigenetic age may differ from their biological age, and it may even differ between different organs in their body; however, it is clear that the aging of the cellular epigenome is a key player in the age-related decline of cell and organ health and function. Therapies that target age-related changes in the epigenome thus have widespread rejuvenation potential and could redefine the aging landscape.
To understand how to turn back the epigenetic clock, one can look to nature’s solution. With reproduction, the epigenetic slate is wiped clean. During the embryonic transition, the epigenome is reset via cellular reprogramming, allowing for the offspring to develop from completely new age 0 cells. This aggressive reprogramming to age 0 is appropriate for the formation of a new individual but would not be desirable for an anti-aging therapeutic as cells would lose their identity and organization, quashing cellular and organ function. It would be most beneficial to revert cells to a time when they were still properly functional.

In normal conditions, the epigenome is able to respond to environmental cues to allow for changes in gene expression that maintain normal cellular function. As the cell ages, the epigenome loses this flexibility, increasing the likelihood of inappropriate gene expression. We can measure this rigidity to get an approximation of cell age using an epigenetic clock.
A person’s epigenetic age may differ from their biological age, and it may even differ between different organs in their body; however, it is clear that the aging of the cellular epigenome is a key player in the age-related decline of cell and organ health and function. Therapies that target age-related changes in the epigenome thus have widespread rejuvenation potential and could redefine the aging landscape.
To understand how to turn back the epigenetic clock, one can look to nature’s solution. With reproduction, the epigenetic slate is wiped clean. During the embryonic transition, the epigenome is reset via cellular reprogramming, allowing for the offspring to develop from completely new age 0 cells. This aggressive reprogramming to age 0 is appropriate for the formation of a new individual but would not be desirable for an anti-aging therapeutic as cells would lose their identity and organization, quashing cellular and organ function. It would be most beneficial to take cells back in time to that when they were fully functional, like in early to mid-adulthood.


Ananda Labs has pioneered the development of the “Rejuvenation-based Placental Epigenetic Reprogramming” (RPER) technology, utilizing patented placental and embryonic factors. This technology treats cells with a combination of reprogramming factors that shock them out of their aged, dysfunctional states, and promote a more youthful, tightly regulated epigenome. The RPER involves the use of reprogramming factor RNA encapsulated in a fatty membrane casing or clinical grade adeno-associated viruses (AVVs), which can be specifically directed to particular cellular populations. This targeting mechanism ensures that the RNA/AAV is efficiently absorbed and utilized by the body’s cells. Since the RPER is based on RNA/non integrating technology, it does not alter the DNA but instead enhances its expression capability.



Lipid-coated RNAs are injected and enter target cells.
The cellular protein-making machinery begins reading the RNAs, producing the reprogramming factors within the cell.
Once produced, the factors are then able to restore the cellular epigenome. These factors are cpmpletely used by the cell or degraded within 24 hours, but their effects are longer lasting.
Cells and organs age at varying rates, largely influenced by the condition of their epigenome. Therapeutic approaches that aim to restore the epigenome offer a promising route to enhance the functionality of aged cells across various types, potentially revolutionizing the healthcare landscape for America’s expanding elderly population.
The “Rejuvenation-based Placental Epigenetic Reprogramming” (RPER) technology represents a groundbreaking advancement in this area. Encased in lipid membranes tailored to target specific cell populations, or utilizing clinically graded AAVs, this therapy rejuvenates the epigenome, thereby restoring more youthful cellular function in a precisely timed manner. By reversing the epigenetic markers of aging, the RPER effectively extends the health span, reducing the impact of age-related diseases and improving quality of life.
Next page: The problem of aging
Request password by email: info@ananda-labs.com